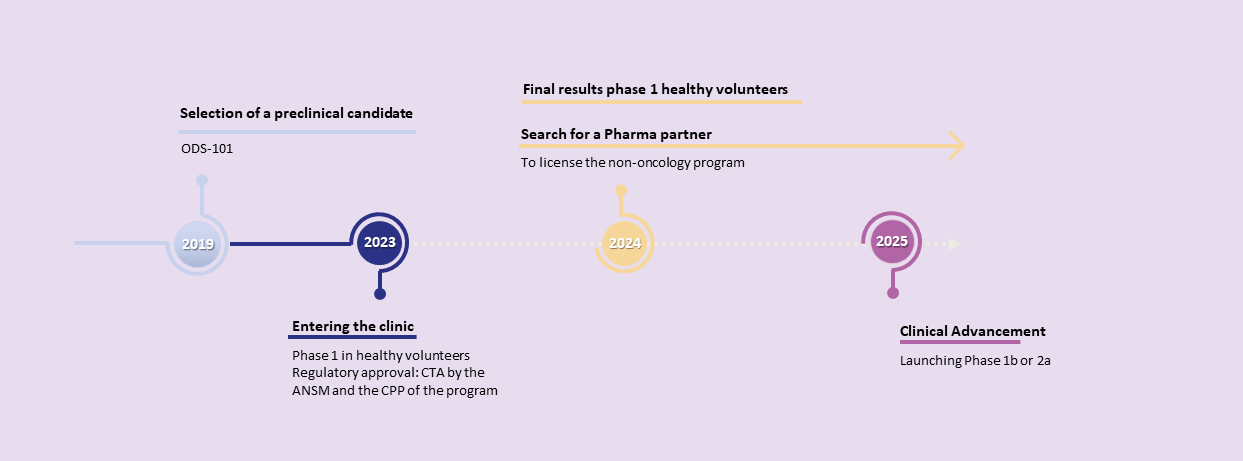
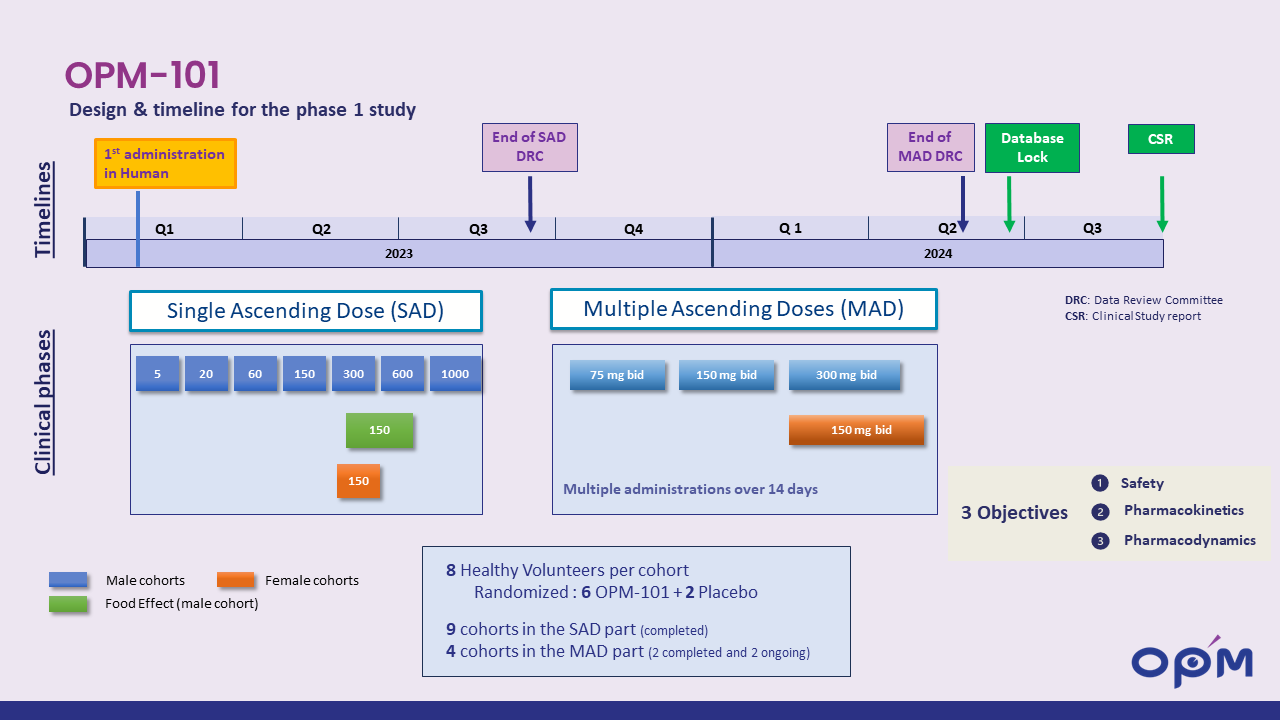
Unlocking Potential in IBD Treatment: Harnessing the power of cutting-edge science, OPM-101 stands as a potential best-in-class inhibitor of the RIPK2 target, and has recently completed the active part of Phase I clinical trials. This groundbreaking molecule holds promise as a game-changer in addressing the complexities of inflammatory bowel disease (IBD), both as a standalone and combination product.
A Vision for Healing: RIPK2, a pivotal kinase within the innate immune system, emerges as a promising candidate in the therapeutic arsenal against IBD, an autoimmune condition rooted in immune system dysregulation. By targeting this key player, OPM-101 offers a novel therapeutic approach to combatting IBD, with initial focus on Crohn’s disease and ulcerative colitis.
Shaping Tomorrow’s Healthcare: At the core of its mechanism lies the recognition of RIPK2’s role in the immune response to bacterial infections, particularly in safeguarding the colon.
By targeting the hyperactivity of this complex, OPM-101 holds the potential to alleviate the burden of inflammatory diseases, with estimated blockbuster potential exceeding $1 billion USD by 2030.
OPM announced positive results of its phase 1 in healthy volunteers with OPM-101: strong target engagement with excellent safety profile : read more
Start of phase 1b/2a clinical trial planned for the fourth quarter of 2024
OPM’s best-in-class RIPK2 inhibitor, OPM-101, has just finished Phase I clinical trials and could offer a novel approach in IBD treatment.
OPM-101 phase I healthy volunteers
Join us in rewriting the narrative of IBD treatment with OPM-101, our flagship asset to innovate the healthcare landscape in IBD.
A promising target to fight inflammatory bowel diseases